We have been talking about zuranolone for a while and are eagerly awaiting its arrival. If you have not heard about it yet, zuranolone is similar to brexanolone, which was approved in 2019 by the FDA for the treatment of PPD. Like brexanolone, zuranolone is a neuroactive steroid with antidepressant activity and a novel mechanism of action as positive allosteric modulators of GABA-A receptors. The big difference between the two is that zuranolone is available as a pill, while brexanolone is administered as an intravenous infusion. This is a huge advantage when it comes to ease of use and accessibility.
Another Study Yields Positive Result
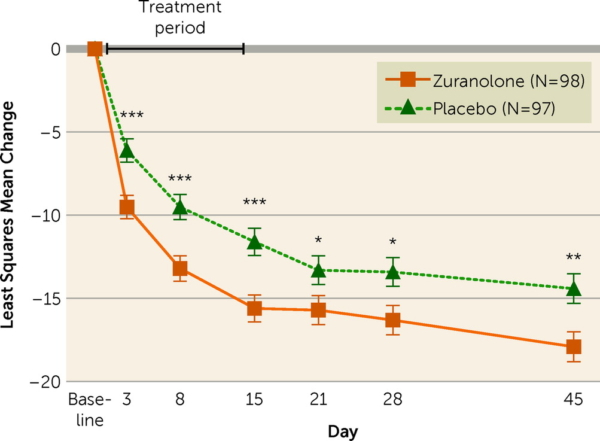
Thus far there have been several randomized controlled trials supporting the efficacy of zuranolone for the treatment of postpartum depression (PPD). In the most recent study, a double-blind phase 3 trial published int the American Journal of Psychiatry, women with severe PPD were randomized to receive zuranolone (50 mg/day) or placebo for 14 days. Depressive symptoms were assessed at baseline and on days 3, 28, and 45 the 17-item Hamilton Depression Rating Scale (HAM-D).
Among the 196 patients randomized (zuranolone, N=98; placebo, N=98), 170 (86.7%) completed the 45-day study. Treatment with zuranolone resulted in statistically significant reductions in depressive symptoms at day 15 compared to placebo (change from baseline in HAM-D score, -15.6 vs. -11.6). Looking at the proportion of patients achieving HAM-D response, zuranolone was superior to placebo starting at day 3, and this trend was observed at every subsequent study visit. The proportion of patients achieving HAM-D response at day 15 was significantly higher in the zuranolone group compared to the placebo group (57.0% vs. 38.9%; odds ratio=2.02, 95% CI=1.11, 3.67).
Looking Forward
This is very exciting. Zuranolone would represent a new option for the treatment of PPD, a rapidly acting antidepressant with a good safety profile. In clinical trials, zuranolone has generally been well-tolerated; the most common adverse events included headache, dizziness, and sedation. In addition, individuals treated with zuranolone experience reductions in anxiety and insomnia, symptoms commonly associated with PPD.
There are still some questions. Women participating in zuranolone clinical trials were asked to forgo breastfeeding while taking zuranolone. At this point we have no information regarding the secretion of zuranolone into the breast milk or the effects of zuranolone in the breast milk on the nursing infant. Nor do we know if zuranolone affects breast milk production. For many women, having to interrupt or avoid breastfeeding while receiving treatment for PPD may be a significant deterrent.
Another big question is related to the duration of the response. Women taking zuranolone continued to experience a reduction in depressive symptoms 30 days after taking the last dose of medication. With traditional antidepressants, we generally recommend that women continue treatment with an antidepressant for 6-12 months, or longer in women with recurrent depression, in order to minimize the risk for relapse. Will women experience recurrent symptoms at some point after a 15-day course of zuranolone? Will they need some sort of maintenance antidepressant therapy? Will they need zuranolone boosters? We don’t yet know, but information regarding long-term outcomes will be collected as we gain expertise regarding this new medication.
Although further study is required and zuranolone may not represent a first-line treatment for all women with PPD, there are many women who will benefit from having another treatment option, including women with severe symptoms and those unable to access long-term psychiatric care.
The FDA is expected to make a decision on zuranolone by August 5th.
Ruta Nonacs, MD PhD
Deligiannidis KM, Meltzer-Brody S, Maximos B, Peeper EQ, Freeman M, Lasser R, Bullock A, Kotecha M, Li S, Forrestal F, Rana N, Garcia M, Leclair B, Doherty J. Zuranolone for the Treatment of Postpartum Depression. Am J Psychiatry. 2023 Jul 26.







Leave A Comment